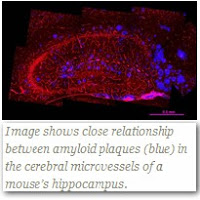
Most researchers think amyloid plaque is the culprit behind Alzheimer’s. They know it damages brain cells. Now they are finding it also accumulates in blood vessels. Learn how it accumulates, about the damage it causes and what to do to prevent it.
NEW YORK — A team of researchers at Weill Cornell Medical College has discovered that amyloid peptides are harmful to the blood vessels that supply the brain with blood in Alzheimer’s disease — thus accelerating cognitive decline by limiting oxygen-rich blood and nutrients. In their animal studies, the investigators reveal how amyloid-ß (also known as plaque, amyloid or amyloid-beta) accumulates in blood vessels and how such accumulation and damage might be ultimately prevented.
Mixed Dementia
The results may shed some light on a common phenomenon called “Mixed Dementia”, which refers to a diagnosis of BOTH Alzheimer’s disease AND vascular dementia. This new discovery may explain why vascular dementia, caused by problems with blood supply in the brain, is so common in people with Alzheimer’s.
The study, published in the online edition of the Proceedings of the National Academy of Sciences (PNAS), is the first to identify the role that the innate immunity receptor CD36 plays in damaging cerebral blood vessels and promoting the accumulation of amyloid deposits in these vessels, a condition known as cerebral amyloid angiopathy (CAA).
Importantly, the study provides the rational bases for targeting CD36 to slow or reverse some of the cognitive deficits in Alzheimer’s disease by preventing CAA.
“Our findings strongly suggest that amyloid, in addition to damaging neurons, also threatens the cerebral blood supply and increases the brain’s susceptibility to damage through oxygen deprivation,” says the study’s senior investigator, Dr. Costantino Iadecola, the Anne Parrish Titzell Professor of Neurology at Weill Cornell Medical College and director of the Brain and Mind Research Institute at Weill Cornell Medical College and NewYork-Presbyterian Hospital. “If we can stop accumulation of amyloid in these blood vessels, we might be able to significantly improve cognitive function in Alzheimer’s disease patients. Furthermore, we might be able to improve the effectiveness of amyloid immunotherapy, which is in clinical trials but has been hampered by the accumulation of amyloid in cerebral blood vessels.”
Mounting scientific evidence shows that changes in the structure and function of cerebral blood vessels contribute to brain dysfunction underlying Alzheimer’s disease, but no one has truly understood how this happens until now.
In the study, the research team — which also includes investigators from the Mayo Clinic in Florida, the McLaughlin Research Institute in Montana and The Rockefeller University — used mice that were genetically modified to develop amyloid in their brain and blood vessels, but in which the CD36 receptor was eliminated. They demonstrated that mice lacking CD36 have less buildup of amyloid in cerebral arteries (CAA) even if they have massive amyloid buildup in their brain tissue (amyloid plaques).
“Remarkably, mice lacking CD36, in which only CAA is reduced, perform significantly better in cognitive tests than do mice with intact CD36,” says the study’s first author, Dr. Laibaik Park, an assistant professor of neuroscience in the Brain and Mind Research Institute.
“In essence, reduced amyloid burden in cerebral blood vessels, or CAA, was able to preserve cognitive function despite the buildup of amyloid plaques in the brain tissue,” says Dr. Iadecola, who is also a neurologist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. “These findings indicate that clearing the amyloid from cerebral blood vessels might be of tremendous benefit to patients with Alzheimer’s disease. These conclusions are based on mice studies, and mice are not humans, of course, but we have a very exciting new direction to explore in our search for Alzheimer’s disease therapies.”
Scavenger Molecule Response Damages Blood Vessels
CAA is already known to be a major cause of brain dysfunction and hemorrhage from weak, damaged brain arteries in some elderly patients, but no one has identified how it occurs. It is also not clear how many older adults suffer from CAA because there is no way to make a clear diagnosis of the condition, unless sophisticated brain imaging studies are performed. But it is believed that this condition is widespread and that CAA, either in association with Alzheimer’s disease or independent of it, is a major cause of cognitive decline in the elderly.
The human brain normally produces the amyloid-β peptide as part of neuronal function, but these peptides are routinely cleared from the brain, in large part, through the blood vessels. However, in most Alzheimer’s patients, the brain’s ability to clear amyloid-β is impaired and, consequently, one type of amyloid-β (Aβ42) accumulates in amyloid plaques and another type (Aβ40) collects in brain arteries, resulting in CAA.
The research team found that CD36, a protein located on the surface of immune cells and in blood vessels, is key to the buildup of Aβ40 in blood vessels. The protein is part of the innate immune system; its function is to act as a sensor to detect molecules that represent a danger to the host. Some of these molecules are derived from invading organisms, such as infectious agents, but some are produced by the body, such as amyloid peptides that, in excess amounts, could become toxic.
“CD36 is a scavenger protein that binds threatening molecules and activates a host of cellular responses designed to get rid of the threat,” Dr. Iadecola says. “Such responses include ramping up inflammation and producing free radicals, both aimed at neutralizing the offenders. However, in the case of amyloid-β, inflammation and free radicals damage brain blood vessels and prevent the efficient clearance of the peptide through these vessels. This, in turn, sets up a vicious circle that favors the vascular accumulation of the amyloid-β peptide and promotes CAA.”
Dr. Iadecola and his colleagues say it may be possible to design new drugs that bind to CD36 on the precise site on the protein’s structure that amyloid-β sticks to, thus blocking the deleterious effects of receptor activation. “We now know how it occurs, and so now we have a new target,” he says.
MORE INFORMATION:
This research study was funded by grants from the National Institutes of Health, the American Heart Association, and the Alzheimer’s Association.
Study co-authors include, Dr. Laibaik Park, Joan Zhou, Dr. Ping Zhou, Dr. Sleiman El Jamal, Dr. Joseph Pierce, Andrea Arreguin, and Dr. Josef Anrather from Weill Cornell Medical College; Rose Pistick and Dr. George A. Carlson from McLaughlin Research Institute in Great Falls, Montana; Linda Younkin and Dr. Steven G. Younkin from the Mayo Clinic in Jacksonville, Florida; and Dr. Bruce S. McEwen from The Rockefeller University.
Weill Cornell Medical College
Weill Cornell Medical College, Cornell University’s medical school located in New York City, is committed to excellence in research, teaching, patient care and the advancement of the art and science of medicine, locally, nationally and globally. Physicians and scientists of Weill Cornell Medical College are engaged in cutting-edge research from bench to bedside, aimed at unlocking mysteries of the human body in health and sickness and toward developing new treatments and prevention strategies. In its commitment to global health and education, Weill Cornell has a strong presence in places such as Qatar, Tanzania, Haiti, Brazil, Austria and Turkey. Through the historic Weill Cornell Medical College in Qatar, the Medical College is the first in the U.S. to offer its M.D. degree overseas. Weill Cornell is the birthplace of many medical advances — including the development of the Pap test for cervical cancer, the synthesis of penicillin, the first successful embryo-biopsy pregnancy and birth in the U.S., the first clinical trial of gene therapy for Parkinson’s disease, and most recently, the world’s first successful use of deep brain stimulation to treat a minimally conscious brain-injured patient. Weill Cornell Medical College is affiliated with NewYork-Presbyterian Hospital, where its faculty provides comprehensive patient care at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. The Medical College is also affiliated with the Methodist Hospital in Houston. For more information, visit weill.cornell.edu.










Can these changes be seen on MRI?
Some of the time. See Section 5 of
http://stroke.ahajournals.org/content/42/9/2672.full
There are also some genetic tests that may be helpful if familial CAA is suspected.
http://stroke.ahajournals.org/content/40/9/3022.full
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3714437/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4004372/
Diane, this study was done in mice. Mice don't get Alzheimer's. Ergo, the relevance to humans is unknown. Even if there is relevance, they are saying they have identified a biomolecule that could be a useful target for innovative drugs to treat CAA and/or comorbid CAA and Alzheimer's. I.e., they haven't developed novel drugs for this target yet, let alone shown that they might be effective in mice or men. This study is of academic interest, not of practical interest to caregivers and patients.
Moreover, the relationships between CAA and Alzheimer's are very complex and not yet at all well understood. The Abeta that forms plaque in blood vessels in CAA is not the same Abeta that forms plaque in the spaces between the brain's nerve cells.
CAA itself is very common and often asymptomatic. When it does cause symptoms, the most common clinical presentation is brain bleed stroke (CAA-ICH). Alternatively, CAA may co-exist with a neurodegenerative disorder, and is considered to be a risk factor for developing Alzheimer's or vascular cognitive impairment, and may contribute to their pathology via, e.g., microbleeds and microinfarcts. Brain bleed stroke per se is not necessarily involved.
See: http://jnnp.bmj.com/content/83/2/124.full
Ok…..so what do we do?
That was my exact reaction.
Take a look at the "LIBRARY" box in the right column. It offers lots of things to do, with sections on Treatment, Clinical Trials, Prevention, Therapy and more. The article above is in the "Research" section. It discusses a discovery that reveals a key understanding of the Alzheimer's problem and a lot of people want that understanding.
One step would be to consider using intensive near infrared light stimulation of the cortex which has been shown to reduce Ab42 by as much as 40% in animal studies. check out http://www.quietmindfdn.org/node/80