
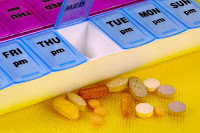
Caregivers Delight: 31-Day Pill Organizer, 4 Times a Day
PRODUCT OF THE WEEK: Caregivers love this pill box, as do patients and professionals. Simply fill it once a month. Roomy, simple, ingenious.

PRODUCT OF THE WEEK: Caregivers love this pill box, as do patients and professionals. Simply fill it once a month. Roomy, simple, ingenious.

The first doses of the newly approved Alzheimer’s drug “Leqembi” were administered in Louisville. More than 100 people have signed up since then. See why.

MEDICATION: Razadyne (generic galantamine) is an FDA-Approved daffodil extract for Alzheimer’s. Learn how it significantly lowered mortality & eased cognitive decline.

Biogen reprioritize resources allocated to ADUHELM® to advance LEQEMBI®.

MEDICATION VIDEO: Aricept® (generic DONEPEZIL) is the world’s most popular drug for Early-stage Alzheimer’s. Does it help in Advanced-stage Alzheimer’s?

CLINICAL INERTIA is when it’s much easier to start someone on a medication, and keep them on, than to take them off. Find out why most advanced dementia patients receive too many expensive, questionable medications.

At long last, we finally have a disease-modifying drug for Alzheimer’s. The FDA recently approved a new drug that promises to slow the progression of the disease. Only five Alzheimer’s treatments have been approved by the FDA up until a decade ago, and this is only the second to address the progression of the disease.

FDA-approved ADLARITY is the once-weekly donepezil patch. See how it compares to donepezil / Aricept / Ebixa tablets for Alzheimer’s.
Swedish researchers find that cholinesterase inhibitors provide cognitive benefits and reduce mortality for up to five years after an Alzheimer’s diagnosis. One medication significantly reduced the risk of developing severe dementia.

Now, a third new Alzheimer’s drug expected to be approved by the Food and Drug Administration (FDA),. The field of new drugs is beginning to show progress in the fight to slow the disease.

DIAGNOSIS VIDEO + ARTICLE:
Country music legend Kris Kristofferson Alzheimer’s misdiagnosis ruined his career. Turned out he had Lyme disease. Learn about his remarkable recovery and the signs to look for.

DIET: MAGNESIUM appears to do more to maintain brain health, prevent cognitive impairment (CI), and fight Alzheimer’s Disease (AD), than previously thought. Learn how.

VIDEO + ARTICLE: People who get more REM sleep get less dementia. REM sleep is when dreaming occurs. Learn about the stages of sleep and their dementia links.

An intriguing study of 120 grandmothers might surprise you. Doctors know socially engaged people have better cognition and less dementia. But can a person get too much of a good thing? What’s the right balance?

If you couldn’t see your mashed potatoes, you probably wouldn’t eat them. That’s why what “The Red Plate Study” found was astonishing! Alzheimer’s patients eating from red plates consumed 25 percent more food than those eating from white plates. Find out why.

Enjoy this great duet between a musician with dementia and his son. A triumph of spirit over Alzheimer’s! Sing-a-long if you like!

It looks like a sneeze cannot give anyone Alzheimer’s. While Alzheimer’s abnormal disease proteins do spread from cell-to-cell, they are not “infectious”. Check out the facts.
No spam, only news and updates.



This site was inspired by my Mom’s autoimmune dementia.
It is a place where we separate out the wheat from the chafe, the important articles & videos from each week’s river of news. Google gets a new post on Alzheimer’s or dementia every 7 minutes. That can overwhelm anyone looking for help. This site filters out, focuses on and offers only the best information. it has helped hundreds of thousands of people since it debuted in 2007. Thanks to our many subscribers for your supportive feedback.
The site is dedicated to all those preserving the dignity of the community of people living with dementia.
Peter Berger, Editor