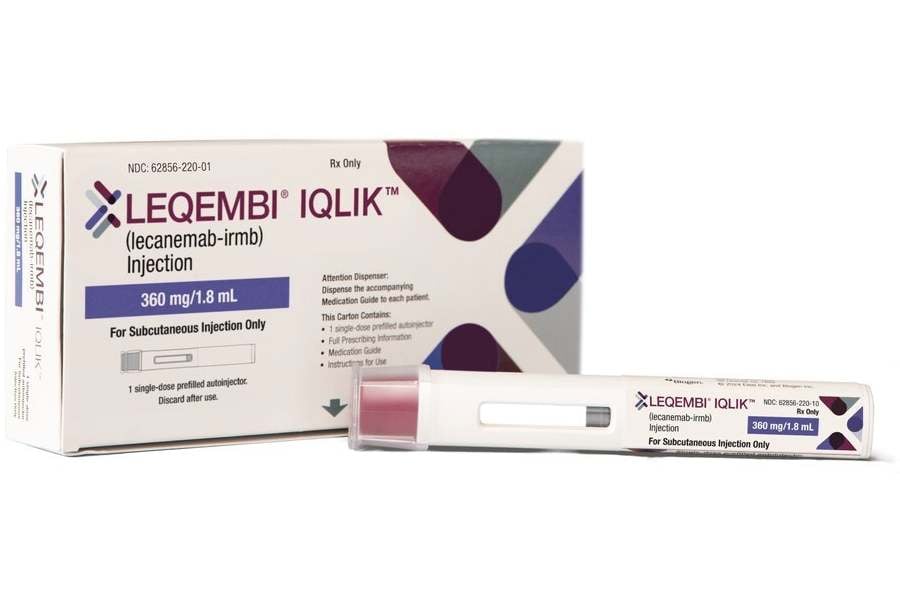
A new version of Leqembi (lecanemab)—called Leqembi IQLIK—has been approved in the United States as a once-weekly injection under the skin. It offers a simpler maintenance option for those who have completed their initial infusion phase of treatment.
What’s the Change?
Until now, patients who finished the first 18 months of Leqembi therapy continued receiving IV infusions at regular intervals. The new form, Leqembi IQLIK, can be injected under the skin just once a week.
This means the maintenance dose can now be taken at home or in a more comfortable setting, instead of at an infusion clinic.
Bottom line: patients who have completed their initiation phase may now choose this easier, less invasive option.
Why This Matters for You and Your Loved Ones
This shift is more than a change in delivery method. It can reduce treatment burden, travel, and time spent in medical facilities.
- Greater convenience — fewer clinic visits and shorter administration times.
- Enhanced access — more people can stay on treatment, especially in areas with limited infusion centers.
- Support and guidance — nurse educators, injection-tracking tools, and assistance programs are being offered to help patients transition smoothly.
For many caregivers, this can mean less time on the road and more quality time at home.
What We Know — and What We Don’t Yet
Safety and Side Effects
In clinical testing, local skin reactions such as redness or itching were relatively mild. Rates of amyloid-related imaging abnormalities (ARIA) were similar between the IV and subcutaneous forms. No new major safety issues were found.
However, researchers are still watching long-term data to learn how well this method performs across broader patient populations. Cost, coverage, and patient adherence will also shape how widely it’s used.
Who Qualifies — and What to Ask Your Doctor
To be eligible for the new subcutaneous maintenance option:
- You must have completed the initial 18-month infusion phase.
- You should be in the early stage of Alzheimer’s (mild cognitive impairment or mild dementia).
When you meet with your neurologist, consider asking:
- Am I eligible to switch to the weekly injection?
- How does the injection compare with the IV version?
- Will my insurance cover it?
- What training will I receive to give the injections safely?
- What monitoring will continue to be required, such as MRI scans?
What You Can Do Next
- Discuss options with your specialist to see if you qualify for the new maintenance dose.
- Ask about patient support programs that offer training, reminders, and assistance.
- Stay informed — future updates may expand who can begin treatment directly with injections.
- Engage with caregiver communities to share experiences and learn from others on Leqembi therapy.
Why This Move Is Significant
Leqembi IQLIK is the first anti-amyloid Alzheimer’s treatment in the U.S. to offer a home-based maintenance injection.
For many families, this change represents more than convenience — it offers a tangible step toward living better with Alzheimer’s, with less time spent in clinics and more time focused on life.