Rudolph E. Tanzi, director of the genetics and aging research unit at Massachusetts General Hospital, professor of neurology at Harvard Medical School and a towering figure in the field of Alzheimer’s research, refuses to play the piano. (Continued below video…)
Yes, he’s an exuberantly dedicated musician who seriously considered a musical career before going into science. He’s played keyboards with the rock band Aerosmith and jammed on “The Tonight Show with Jay Leno.” He practices every day at home on his handmade Bösendorfer concert grand.
But the old piano in the laboratory lounge near his office? By Tanzi’s reckoning, it has the instrumental equivalent of a neurodegenerative disease. “It sounds terrible,” he says. “Tinny, balky, out of tune. I won’t play it.” Please? Just a few bars? “I have my standards,” he mutters. “I don’t want to embarrass myself.”
Finally, he sits down with a sigh and starts in on the jazz classic, “‘Round Midnight.” All that warm-up whining and the ballad sounds wonderful, with elegant harmonies and a spare, inventive baseline. He moves on to Miles Davis, Billy Joel. Scientists and students from elsewhere on the floor stream into the lounge to listen, and when Tanzi finishes they burst into applause. Tanzi, who is 57, looks happy, boyish and maybe relieved. From a tattered piano he’s plucked magic rabbits of song.
Some Kind of Genius
“Rudy is some kind of genius,” says his close collaborator Doo Yeon Kim, who works down the hall. Musically, scientifically, Kim says, “Rudy always has big ideas, always wants to try new things.” Kim, 45, smiles often, speaks with a strong Korean accent and seems perpetually ready to break into a run. He considers himself a nitty-gritty, flask-and-beaker sort of guy. “I focus on the science,” he says. “Rudy’s role is the vision, mine is the details.”
The pair’s contrapuntalism has proved a runaway hit. Tanzi and Kim have devised a revolutionary tool for tackling Alzheimer’s disease, the world’s leading cause of senile dementia and a medical crisis that looms ever larger as the teeming throngs of baby boomers lurch into old age. Reported late last year to international acclaim in the prestigious journal Nature, the new technique, an innovative type of cell culture, is considered the most persuasive and useful laboratory model yet invented of the neurodegenerative disease. It offers researchers a chance to both track the course of Alzheimer’s in unprecedented biochemical and genetic detail, and to quickly and cheaply test thousands of potential treatments that might block or at least slow its malign progress. “It’s a fantastic model with great potential for testing new drugs,” says Sangram Sisodia, a professor and Alzheimer’s researcher at the University of Chicago. “It’s the kind of golden opportunity we haven’t seen before.”
Alzheimer’s-In-A-Dish
Nicknamed “Alzheimer’s in a dish,” the new technique features colonies of genetically manipulated human brain cells that grow in three dimensions in a gooey gel. As the days pass, the cells start displaying the two most salient hallmarks of Alzheimer’s disease: plaques and tangles. Forming around and between the cells, the microscopic plaques consist of cast-off protein fragments called amyloid-beta and are as tough and unyielding as the nubs in a turkey burger, while the similarly stiff and tiny tangles develop inside the brain cells and look like twisted pieces of wire. Plaques and tangles are the very same diagnostic defects that the German neurologist Alois Alzheimer observed more than a century ago as he examined under the microscope the autopsied brains of patients who suffered from the disease that bears his name. Until now, however, scientists had not managed to generate both elements of the disorder in a single laboratory model—not in cells proliferating in petri dishes, not in genetically engineered mice (which form only plaques, not tangles). “I’m very enthusiastic,” says Sam Gandy, director of the Mount Sinai Center for Cognitive Health in New York. “We’re finally able to get key features of the human pathology that we haven’t been able to recapitulate in mice. It’s a powerful system.”

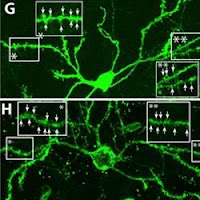
ABOVE: In Tanzi and Kim’s lab, brain cells growing in 3-D in a dish harbor genes for familial Alzheimer’s disease. Each of these microscope images shows a different level of focus on a neuron cluster (green) and toxic amyloid plaque (yellow/orange). (IMAGE SOURCE: Dr. Doo Yeon Kim and Dr. Rudolph Tanzi, Massachusetts General Hospital)
Best Decision
The breakthrough work serves as yet another highlight in Tanzi’s ridiculously fruitful career, in which he has helped hunt down and isolate almost all of the major genes now known to be associated with Alzheimer’s disease. The development also tags Kim as a rising star in a fiercely competitive field, and clinches his conviction that leaving the security and familiarity of South Korea years ago was the right thing to do. “My wife joked at the time, if we’re going to the U.S., why not Hawaii? The weather is much better there than in Boston,” Kim says. “But at Harvard, you feel like you’re in the center of everything.” His wife, Dong Eun Lee, has a good job as a pharmacist. His daughter Helena loves being an American high-school student, with the freedom to cross “pharmacist” and “scientist” from her list of professional aspirations.
The path to Alzheimer’s in a dish was often grueling, switchbacking and potholed with despair, but in the end, Tanzi says, “the data spoke for themselves, and even my rivals were impressed.” And for all Kim’s claims about playing i-dotter to Tanzi’s poetry, a crucial conceptual leap came not from Rudy but Doo.
“Coming here,” Kim says, “was the best decision I ever made.”
New Treatments
The need for new treatments is dire. An estimated seven million Americans are stricken with Alzheimer’s disease, and that figure is expected to quadruple in the next 30 years. Most are over 65 and suffer from a late-onset form of the disease, the result of multiple and still-mysterious slings and insults that take decades to decommission the brain; but a small proportion, roughly 5 percent, are the victims of hereditary Alzheimer’s disease, carrying one of several rare genetic mutations that can cause dementia by age 50 or even younger—the kind of personal apocalypse beautifully portrayed by Julianne Moore in the movie “Still-Alice”. The direct costs of caring for the national dementia burden are enormous, about $225 billion a year today and projected to hit $1 trillion annually by 2050. Yet Alzheimer’s patients can’t do without care: The disease progresses from the early stages of chronic forgetfulness, repeating questions, losing things and growing anxious and irritable, to escalating incompetence at everyday tasks like driving or finding your way home, maybe turning paranoid, stricken by delusions that your loved ones are stealing from or cheating on you, to needing help bathing, grooming, going to the toilet or eating. “It takes eight to ten years, on average, but eventually the patient is in a vegetative state, unable to walk or talk,” says R. Scott Turner, director of the Memory Disorders Program at Georgetown University.
“If we don’t do something about this, it’s going to cripple us,” says Anne B. Young, the former chief of neurology at Mass General, “and those who don’t get Alzheimer’s disease will be impacted just as much as those who do.”
Talent + Attention to Detail
Paradoxically or appropriately, Tanzi is renowned among his peers for his exceptional memory. “Rudy’s got one of the most detail-oriented memories I’ve ever known,” says Wilma Wasco, a neurogeneticist at Harvard. “He can remember papers he read 25 years ago, who the authors were, what they found, what the footnotes said—which is really not typical.” Tanzi honed his retentive skills in childhood, while working for his parents’ medical transcription service in Cranston, Rhode Island. “I would memorize all these medical terms,” he says. “That’s what got me interested in medicine and science.”
He also fell in love with music. “At 9 years old I started playing the accordion, like a good Italian boy,” he says. He improved rapidly. His father bought him a bigger accordion. A year or two later, his father asked, So, you still like the accordion? Yes, young Rudy said. “Then he asked, what do you think, should I get you a monkey and a cup to go with it?” At his father’s prodding, Rudy branched out to other keyboards, and to jazz. He took up piano and mastered the Hammond organ, which is an electronic version of a pipe organ and very difficult to play. “It’s really weird to hear somebody as good as he is on the Hammond organ,” says Joe Perry, the lead guitarist in Aerosmith. “I love jamming with him. He inspires me to try new things on the guitar.”

Tanzi, who has recorded with Aerosmith, accompanies the band’s lead guitarist,
Joe Perry at a 2012 charity concert. (Courtesy Rudolph Tanzi)
At the University of Rochester, Tanzi secured bachelor’s degrees in both microbiology and history, writing his history thesis on Franz Mesmer, an 18th-century German doctor who promoted the idea of “animal magnetism” and from whom we derive the word mesmerize. Tanzi remains fascinated—mesmerized?—by alternative ways of interpreting reality. He regularly rereads the mystical works of Carlos Castaneda. He meditates, practices lucid dreaming and collaborates with the new-age superstar Deepak Chopra. They’ve co-written two books that blend popular science and self-help—Super Brain and the just-published Super Genes—and they travel the world as a team, talking about the nature of consciousness. “We do the dog and pony show together,” Chopra says. “He’s a very reflective thinker, and more open than most scientists to holistic ideas. We’ve become buddies.” Yet make no mistake. When it comes to his research, Chopra says, “Rudy is very meticulous, and very careful in his language. He’s an extremely ambitious scientist.” Of Tanzi’s many pursuits, Chopra says, “science is his number one love.”

Harvard Medical School
As a graduate student at Harvard Medical School, Tanzi worked with the geneticist James Gusella (“one of my heroes,” Tanzi says), and in 1983 their team gained international acclaim for becoming the first scientists ever to locate the approximate genetic address of a disease trait by fishing at random through the bewildering megalopolis of the human genome with tagged bits of DNA. Using an approach that has since become standard among gene mappers, Gusella, Tanzi and their colleagues pinned the source of Huntington’s disease, the inherited neurodegenerative disorder that killed folk singer Woody Guthrie, to a spot on chromosome 4, out of the 23 pairs of chromosomes that constitute the human genome and that nearly all human cells enfold.
From that heady career kickoff, Tanzi turned his cartographic talents to the deciphering of chromosome 21, which, when inherited in triplicate, causes Down syndrome. On learning that people with Down often end up contracting Alzheimer’s disease as well, Tanzi realized he had found his life’s calling. He would search for the genetic roots of Alzheimer’s, starting with the tantalizing link to chromosome 21. He married an endodontist, they divorced, he married a neuroscientist named Dora Kovacs, his current wife. They have a daughter, Lyla, who’s in second grade. Every weekend he makes her pancakes. Once, when Lyla had an ear infection, he made her a pancake shaped like an ear.
Genetics
Since the late 1980s, Tanzi, his colleagues and his rivals have identified three different genes that, when inherited in mutant form, inevitably result in the early-onset version of Alzheimer’s disease. (One of them is indeed located on chromosome 21, which is why patients with Down syndrome regularly end up bearing an Alzheimer’s defect as well.) None of the mutations found on those three genes are directly involved in the common Alzheimer’s of old age, but because the brains of patients display a similar mosaic of microscopic abnormalities regardless of when the disease strikes, researchers believe that patients who have inherited the familial form of the disease hold clues relevant to all.
By the looks of it, researchers say, the genetic mutations disrupt the brain’s capacity to manage the everyday trafficking and processing of essential proteins. As a result, excess quantities of the amyloid-beta protein, which the brain normally uses to protect itself—perhaps against bacterial infection, Tanzi’s research suggests—do not get flushed away or recycled, but instead gum together into plaques around brain cells. Another protein, called tau, also turns rogue and twists up into tangles inside the neurons. Dendritic connections between neurons wither, short-circuiting thought. Injured brain cells flare up and then collapse, like tiny, dying suns. The brain shrinks by 20, 30 percent. The self follows suit.
But how exactly does the protein misprocessing get started? Are the plaques the worst offenders, or are they a distraction from the real villain, the tangles, or something else altogether? More important, how can the process be stopped? To answer that, researchers needed a good laboratory mimic of the disease, and even with the three disease genes in hand, they still didn’t have that.
Dr. Kim
As a graduate student in cell biology at the Korea Advanced Institute of Science and Technology, among the nation’s most competitive universities, Doo Yeon Kim became fascinated by neurons. “They’re very complicated and very different from other cells of the body,” he says. “I thought, I’ll do basic cell biology on neurons to understand their behavior. I’ll look at neurodegenerative disease to understand how they die.” South Korea had few neuroscientists to work under, but Kim plugged away on his own. Through computer analysis, he identified a gene he thought might play a role in Alzheimer’s. “Somebody told me, oh yes, that’s a gene Rudy Tanzi is studying right now,” Kim says. “Rudy was very popular in South Korea, a real star. I thought maybe I should try to go work with him.” Kim sent Tanzi an email, requesting a position in his lab. Kim didn’t hold much hope. He figured a guy like Tanzi was bombarded by pleas and résumés. “I didn’t think I’d hear from him,” Kim says. “He got back to me in one day. He said, I think you look good.”
A Better Model
Tanzi really wanted a better model for understanding Alzheimer’s. He also wanted proof for his hypothesis that excess amyloid-beta was at the heart of the disease: that it not only caused plaques but, by helping to turn tau protein rotten, it touched off tangles, too. Se Hoon Choi, a postdoctoral fellow in Tanzi’s lab at the time, remembered a meeting when Tanzi said jocularly, Wouldn’t it be nice to show that amyloid secretion causes tau pathology? “Rudy makes a lot of jokes,” Choi says, “but they’re food we can eat.”
Kim wanted to try his hand at modeling Alzheimer’s. He, Choi and Tanzi discussed possible approaches. They decided to use human neurons, a risky approach: Such cells rarely survive in petri dishes for the necessary long haul. Luckily, another young researcher from South Korea, Young Hye Kim (no relation to Doo), would be joining the lab for two years and had a guaranteed job to return to: She could afford to focus on the project without fearing for her professional future should it prove a flop.
The researchers started with human neurons derived from stem cells, spread them in single layers in culture dishes and bathed them in nutrient-packed liquid. Next, they used specially designed viruses to deliver mutant copies of two different familial Alzheimer’s genes into each cell. The neurons thrived. They grew into reliable cell lines. Very nice. But time passed, and the cells weren’t doing anything. No signs of plaques. Not a trace of tangle. “I could tell that Young was getting really depressed,” Kim says. “I’d suggest something, she’d say, Why bother? It won’t make any difference.”
Brainstorm
That’s when Kim had his brainstorm, if you will. Maybe the problem was the liquid medium, he thought. Maybe the need to change it every three days ended up washing away any dubious proteins the cells might be secreting before those proteins had a chance to stick together into plaques. “Doo made a very simple observation,” Tanzi says. “The brain is not made of liquid. It’s a gel.” The researchers moved the cells from dishes to little wells filled with gel. They fiddled with parameters. The cells looked happier than ever, forming feathery dendrites that pulsed with measurable electric signals. Six weeks passed, and the researchers got a jolt of their own.
There, through the confocal microscope, an unmistakable image: The cells had formed plaques. “I couldn’t believe what I was seeing,” Tanzi says. They were ready to publish a paper on their in vitro conjuring of plaques. Two more weeks passed. Young Hye checked the cells, sampled their protein arrays. “She called out to me excitedly,” Kim says. “It was the first and last time that she used my first name.” Doo! Come quick! There are tangles of tau! “It was one of those rare aha! moments in science,” Tanzi says.
Plaques & Tangles
Another triumph soon followed. The researchers showed that if they blocked amyloid-beta output with antibodies, the cells not only failed to form plaques, they didn’t form tangles, either. “They have validated in the best way possible the idea that amyloid abnormality is driving Alzheimer’s disease,” said Dennis Selkoe, another Alzheimer’s researcher at Harvard Medical School.
Now what we need, Tanzi and others believe, are drugs that can modulate amyloid-beta output. Not block it entirely, Tanzi says. “It’s like cholesterol,” he says. “You just want to dial it down.” We need the equivalent of statins, he says—drugs to inhibit plaques in the brain just as statins help clear plaque from your blood vessels. Tanzi is now working with the Cure Alzheimer’s Fund on an initiative that will screen virtually every FDA-approved drug out there. “Whether it’s for asthma or back pain, we can see if it works in our system against plaques and tangles,” he says. “It’s ten times faster and a hundred times cheaper than doing the same tests in mice.”
How to Keep Brains Young
Alzheimer’s in a dish is still new and has yet to make its mark on treatment. In the meantime, for those who seek advice on how to keep their brains young, Tanzi and others agree on these steps: Get plenty of physical exercise. Sleep seven or eight hours a night. “It’s during deep, slow-wave sleep that the brain cleans out the debris,” Tanzi says. Eat a healthful, Mediterranean-style diet. And keep learning, keep building up what Tanzi calls “synaptic reserve.” It’s never too late to learn the piano. You don’t need a Bösendorfer. Any clunker will do.”












The article is very detailed analysis. Tanzi’s extraordinarily successful career has been highlighted by this ground-breaking study, since he was instrumental in locating and isolating nearly all of the essential genes currently thought to be implicated in the disease. Alzheimer’s. Because of this, Kim is now a rising star in a field with a lot of established players.
——–
Happy Wheels