VIDEO+ARTICLE
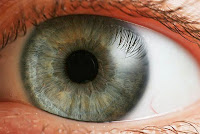
Cognoptix announced strong results in a clinical trial of its SAPPHIRE II eye test. It successfully identified Alzheimer’s via the human eye’s beta-amyloid signature. See how Cognoptix will check if you are clear of Alzheimer’s, right in your doctor’s office.
Cognoptix published strong results of a multi-site clinical trial of its SAPPHIRE II eye test in the peer-reviewed Journal of Alzheimer’s Disease & Other Dementias® (AJA).
“Our vision is to change the course of Alzheimer’s disease by enabling early diagnosis at the point of care,” said Cognoptix President and CEO Paul Hartung.
“We have made great progress, ” Hartung added, “in developing an early-stage, non-invasive diagnostic test for AD designed to allow treatment to start before significant neuronal loss and irreversible brain damage occur.”
By detecting a specific fluorescent signature of ligand-marked A-beta in the supranucleus region of the human lens, SAPPHIRE II achieved a sensitivity of 85% and a specificity of 95% in differentiating 20 patients who were clinically diagnosed with probable AD from a group of 20 age-matched healthy volunteers. In addition, the SAPPHIRE II test showed excellent correlation to PET (positron emission tomography) amyloid brain imaging.
“The easy-to-use SAPPHIRE eye test has demonstrated the clinical potential to remake the paradigm for the way in which Alzheimer’s Disease is currently diagnosed and managed,” said Carl Sadowsky, MD, FAAN, Medical Director, Premiere Research Institute, West Palm Beach, Fla., and a principal investigator in the clinical trial of the SAPPHIRE eye test.
“We are delighted with the results of this feasibility study, which demonstrates the safety and effectiveness of our product, and are honored to have our work recognized in a well-respected, peer-reviewed journal,” said Paul Hartung, President and CEO of Cognoptix. “Our vision is to help change the course of Alzheimer’s disease by enabling early diagnosis at point-of-care.”
MORE INFORMATION:
About Cognoptix
Cognoptix, a privately held medical technology company headquartered in Acton, Mass., is focused on developing and commercializing an in-office, drug/device diagnostic system as an aid in the early detection of Alzheimer’s Disease (AD). Its investors include Inventages Venture Capital, one of the world’s largest life sciences-, nutrition- and wellness-focused venture capital firms; Launchpad Venture Group, a Boston-based angel investment firm that provides funding to early-stage companies; and Maine Angels, accredited private equity investors in promising New England entrepreneurs and companies.
About AJA
American Journal of Alzheimer’s Disease & Other Dementias® (AJA) is for professionals on the frontlines of Alzheimer’s care, dementia, and clinical depression–especially physicians, nurses, psychiatrists, administrators, and other healthcare specialists who manage patients with dementias and their families. This journal is a member of the Committee on Publication Ethics (COPE).