VIDEO+ARTICLE:
Harvard has discovered key capabilities of a gene regulator called “REST”. It seems to offer resistance against the toxic effects of Alzheimer’s. Can scientists block early Alzheimer’s by activating “REST” with drugs?
Why do neurodegenerative diseases such as Alzheimer’s affect only the elderly? Why do some people live to be over 100 with intact cognitive function while others develop dementia decades earlier?
Continued below video…
More than a century of research into the causes of dementia has focused on the clumps and tangles of abnormal proteins that appear in the brains of people with neurodegenerative diseases. However, scientists know that at least one piece of the puzzle has been missing because some people with these abnormal protein clumps show few or no signs of cognitive decline.
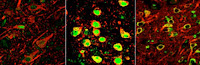
gene regulator called REST, dormant in the brains of young people
(left), switches on in normal aging brains (center) to protect against
various stresses, including abnormal proteins associated with
neurodegenerative diseases. REST is lost in critical brain regions of
people with Alzheimer’s (right).
Credit: Yankner Lab
A Harvard study offers an explanation for these longstanding mysteries. Researchers have discovered that a gene regulator active during fetal brain development, called REST, switches back on later in life to protect aging neurons from various stresses, including the toxic effects of abnormal proteins. The researchers also showed that REST is lost in critical brain regions of people with Alzheimer’s and mild cognitive impairment.
“Our work raises the possibility that the abnormal protein aggregates associated with Alzheimer’s and other neurodegenerative diseases may not be sufficient to cause dementia; you may also need a failure of the brain’s stress response system,” said Bruce Yankner, Harvard Medical School professor of genetics and leader of the study.
“If true, this opens up a new area in terms of treatment possibilities for the more than 5 million Americans currently living with Alzheimer’s disease,” said Yankner, who in the 1990s was the first to demonstrate the toxic effects of amyloid beta, the hallmark abnormal protein in Alzheimer’s.
The results were published Mar. 19 in Nature.
Protection at the end of life
The CDC lists Alzheimer’s disease as the sixth leading cause of death in the United States, and a Mar. 5 paper in Neurology by a group unrelated to Yankner’s argued that it should be ranked third. A 2013 study by the RAND Corporation found that with an estimated annual toll of as much as $215 billion, Alzheimer’s is America’s most expensive disease, costing more than heart disease or cancer.
“Dementia is not an inevitable result of aging,” said Yankner, who is also co-director of the Paul F. Glenn Laboratories for Biological Mechanisms of Aging. “We know it’s possible for the human brain to work normally for a century or more. So a robust mechanism must have evolved to preserve brain function and keep brain cells alive in long-lived organisms like us. We just haven’t learned what that mechanism is.”
Yankner believes REST may be a key piece in the solution to that puzzle. REST first came to his attention when team member Tao Lu, HMS instructor in genetics, flagged it as the most strongly activated transcriptional regulator — a switch that turns genes on or off — in the aging human brain. The team confirmed the finding through biochemical and molecular tests and high-resolution imaging.
The finding surprised him at first because until then, REST’s only known activity in the brain occurred prenatally, when it keeps key genes turned off until progenitor cells are ready to differentiate into functional, mature neurons. REST was believed to wind down in the brain soon after birth. (It stays active elsewhere in the body and appears to protect against several kinds of cancer and other diseases.) When Yankner thought more about it, however, it began to make sense.
“When in a person’s life are brain cells most vulnerable?” he asked. “The first time is during fetal development, when loss of young neurons would be devastating. The second is during aging, when you’re bombarded by oxidative stress and misfolded or aggregated proteins, such as the amyloid beta and tau proteins seen in Alzheimer’s disease. It makes sense that a system would come on at those two times to protect neurons, which are largely irreplaceable.”
Having discovered this possible new role for REST, Yankner and team went on to identify the specific genes REST regulates in aging neurons. They found that REST turns off genes that promote brain cell death and contribute to various pathological features of Alzheimer’s disease, such as amyloid plaques and neurofibrillary tangles, while it turns on genes that help neurons respond to stress.
Lab dish experiments revealed that removing REST made neurons more vulnerable to the toxic effects of oxidative stress and amyloid beta. REST appeared to clear away and protect against the free radicals that result from oxidative stress.
To confirm REST’s role, the team engineered mice that lacked REST only in their brains and watched what happened as they aged.
“The mice were okay as young adults, but as they got older, neurons in the brain started to die in the same places as in Alzheimer’s: the hippocampus and the cortex,” said Yankner. “This suggested that REST is essential for neurons to remain alive in the aging brain.”
Together with HMS associate professor of genetics Monica Colaiácovo, the team also uncovered a REST equivalent in the tiny worm C. elegans. There, too, the REST equivalent was necessary to protect against free radicals and amyloid toxicity. This suggested the protective function is shared across species.
Diverted from its course
Yankner and colleagues further illuminated the relationship between REST and the aging brain through a combination of lab experiments and studies of brain tissue from elderly people with and without dementia.
The team showed that REST was activated in normal aging brains. The brains of people who developed mild cognitive impairment, by contrast, showed an early decline in REST. The affected brain regions of people with Alzheimer’s had hardly any REST left.
“REST loss correlates very closely with memory loss, especially episodic or autobiographical memory, the type that typically declines early in Alzheimer’s,” said Yankner.
Cell culture experiments suggested REST is activated when stressed neurons send signals to one another, and that once REST is created in a neuron’s cytoplasm, it must travel to the nucleus to do its job.
Yankner’s group then found that in Alzheimer’s, REST gets diverted from its journey to the nucleus, becomes engulfed through a process called autophagy and is eventually destroyed.
The team saw the same striking misplacement of REST when they looked at brain tissue from people with other prevalent neurodegenerative diseases involving dementia, including frontotemporal dementia and dementia with Lewy bodies. In all three dementing illnesses, REST had been swept into the cellular trash bins alongside each disease’s abnormal proteins: amyloid beta in Alzheimer’s, tau in frontotemporal dementia and alpha-synuclein in Lewy body disease.
“The prevention of REST from getting to the nucleus may be the earliest phase in the loss of REST function. Our laboratory models suggest that this will make neurons much more vulnerable to a variety of stresses and toxic proteins,” said Yankner.
Uncovering how REST gets activated and misplaced provides new ideas for how to intercept Alzheimer’s. For instance, rather than solely focusing on lowering amyloid beta levels, as clinical trials have done so far without great success, Yankner imagines trying to target REST with drugs such as lithium, which his lab has shown can boost REST function.
REST and dementia-free longevity
Next, Yankner turned to the long-standing puzzle in neurology of how some aging individuals can harbor Alzheimer’s disease pathological changes but never become demented.
The team examined brain tissue gathered as part of the Religious Orders Study and the Rush Memory and Aging Project, both funded by the National Institute on Aging. These long-term studies together follow several thousand aging participants and collect donated tissue after death to better understand normal aging, cognitive impairment and neurodegenerative disease.
The team sorted the samples into two groups. One group had Alzheimer’s pathology and experienced symptoms of dementia. The second group had the same amount of Alzheimer’s pathology but did not become demented. The team found that the group with no dementia had at least three times more REST in the nuclei of their neurons in key brain regions.
“This suggests a person may be able to resist the toxic effects of Alzheimer’s pathology if REST levels remain high,” said Yankner. “If we could activate this stress-resistance gene network with drugs, it might be possible to intervene in the disease quite early.”
“Since Alzheimer’s strikes late in life, delaying the onset of disease by just a few years could have a very substantial impact,” he added.
In additional studies, the team found that REST strongly correlated with increased longevity. REST levels were highest in the brains of people who lived into their 90s and 100s and remained cognitively intact. Levels stayed high specifically in the brain regions vulnerable to Alzheimer’s, suggesting that they might be protected from dementia.
Finally, the team showed that REST increases the expression of several genes known to increase lifespan in model systems of aging.
It remains to be seen how many more pieces will slot in alongside REST in solving the puzzle of aging and dementia. For now, the team’s findings offer new ideas for combating a disease that currently has no treatment.
“I’m sure there is something else at play that hasn’t been seen or measured yet. REST won’t be the end-all. But I think our work will help shift attention to this protective pathway in the aging brain and its role in the prevention of Alzheimer’s and other dementing diseases,” said Yankner.
“It’s a new point of view on the problem.”
Source:
Journal Reference:
- Tao Lu, Liviu Aron, Joseph Zullo, Ying Pan, Haeyoung Kim, Yiwen Chen, Tun-Hsiang Yang, Hyun-Min Kim, Derek Drake, X. Shirley Liu, David A. Bennett, Monica P. Colaiácovo, Bruce A. Yankner. REST and stress resistance in ageing and Alzheimer’s disease. Nature, 2014; DOI: 10.1038/nature13163









It is interesting that professor Yankner , the prestigeous researcher that "in the 1990s was the first to demonstrate the toxic effects of amyloid beta" , now , in 2014, AT LAST , realizes and declared in the text above , based in scientific research, that betamyloid accumulation it is NOT the cause of AD (!) , and suggested too that based in scientific logic , that amyloid accumulation only hastens the LASTS steps of the disease.In summary , the prestigeous researcher professor Yanker ,looks to support that the amyloid hypothesis as the CAUSE of AD it is a big mistake (!) as we can see in the part of the text that I pasted bellow :
"Our work raises the possibility that the abnormal protein aggregates (BETAMYLOID ACCUMULATION) associated with Alzheimer’s and other neurodegenerative diseases may NOT BE SUFFICIENT TO CAUSE DEMENTIA ; you may also need a FAILURE of the brain’s STRESS response system,” … said Yankner"
That it is a declaration to be celebrated , once it can leads to researchers to the "freedom",to search for effective treatments , leaving to the past, the 28 years of the catastrophic failures of the non sense of the abeta hypothesis.
But REST research it is interesting , but still looks to be a small step upstreams to the amyloid accumulation.
But as professor Yankner realizes and declared too , oxidative stress comes first, then , one day , amyloiders researchers will "realizes" that brain energy disorders, mitochondrial disorders, peroxinitrites accumulation are , for sure , the others upstreams disorders, that preceeds the REST disorders in dozens of years.
But to recognize and declare that the amyloyd hypothesis is flawed , that is a great beginning.