UNDERSTANDING ALZHEIMER’S – VIDEO & ARTICLE:
IS IT TIME, after 35 years, for Alzheimer’s researchers to change course? In 1984, the “Amyloid Hypothesis” fingered amyloid-beta plaque as the culprit behind Alzheimer’s. 35 frustrating years later, we haven’t found the cure, yet amyloid remains the #1 suspect. Are we barking up the wrong tree? A new study takes a look at the arguments. Read the pros and cons.
A new study reviews the huge body of research proposing that the accumulation of beta amyloid triggers Alzheimer’s disease in the brain. The study appears in an open access, peer-reviewed title from Future Science Group.
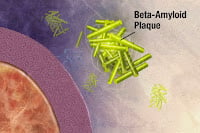
In 1984, a study by Glenner and Wong can be said to have initiated the "Amyloid Hypothesis". It suggested that plaque on the brain made out of beta-amyloid was the trigger behind Alzheimer’s.
Once the target was identified, scientists natually zoomed in on it to try and cure Alzheimer’s.
Continued below video…
The Amyloid Hypothesis
In overly simple terms, the brain contains a large protein called the Amyloid Precursor Protein (APP for short). APP’s function is still unclear. It is expressed and cleared in considerable quantities in neurons, the heart, lung, liver and skin [1].
Aβ stands for amyloid-beta, or amyloid for short, the suspect behind Alzheimer’s. Aβ is a derivative of the longer amyloid precursor protein (APP). So researchers figured the way to cure Alzheimer’s is to block the generation of Aβ through modulation of proteins called secretases. Secretases do the dirty work of cutting harmless APP into brain-killing Aβ. Inhibit the secretases and you get less Aβ. Less Aβ should mean less Alzheimer’s.
What Do We Know After 30 Years?
Secretases: Today, it remains far from certain whether targeting the secretases involved in APP processing will yield the ground breaking therapeutic that is urgently required to treat Alzheimer’s disease (AD). The number of high-profile failures in recent years has severely impacted the confidence of large pharmaceutical companies. A number of companies have scaled back their risk in this field. Nonetheless, the potential rewards for discovering a drug to treat Alzheimer’s prevent a full retreat by key players.
Vaccines: Another approach has been to create an Alzheimer’s vaccine. Attempts to target Aβ through drugs that induce immunity have produced similar negative results leading to recent high profile clinical failures. The two most famous ones were Bapineuzumab, developed by Johnson & Johnson and Pfizer [3] and Solanezumab, developed by Eli Lilly [4]. Both looked promising and then failed their primary end points in Phase III. Yet Eli Lilly has not given up on solanezumab and new-but-different trials are rolling out.
Wrong Road?
The amyloid hypothesis has now been the mainstay of therapeutic research in Alzheimer’s disease for over two decades. The series of high profile clinical failures has inevitably called into question the viability of the hypothesis itself. A number of issues have plagued the amyloid hypothesis since its inception. First, the level of Aβ burden does not often correlate with clinical manifestation of the disease. In several studies, amyloid plaques were apparent in healthy people despite no evidence of cognitive decline [5-8]. However, other investigations have found a much stronger correlation between levels of Aβ, the loss of physical synapses in the brain, and obvious cognitive decline [9,10].
Second, Aβ is complex and comes in many "flavors". The difficulty in isolating the specific neurotoxic species of Aβ and characterizing its effects makes research problematic. Early studies demonstrated that Aβ is toxic, [11] but it was also noted that different preparations result in different potencies of the Aβ peptide [12]. Furthermore, Aβ has several distinct conformations which appear to have different toxic effects on neurons. These include: oligomers composed of 15–20 monomers; small diffusible Aβ oligomers known as ADDLs (Aβ-derived diffusible ligands); and protofibrils (strings of oligomers) [13-16].
Further criticism of the evidence underpinning the amyloid hypothesis revolves around the mice used in the research labs. Today’s mouse-models of Alzheimer’s do not fully recapitulate the disease. When researchers increase Aβ deposition in these mouse-models, there is a lack of coincidental neuronal loss. This is thought to be due mainly to species differences in neuronal susceptibility to Aβ accumulation, a lack of the human tau protein in mice, as well as the lack of a human-like inflammatory response which also plays a pivotal role in the progression of the disease [2].
Cons
Critics of the amyloid hypothesis have argued that it is just too simplistic to focus on this one approach. This zooming in on amyoild by researchers may have diverted attention from other important associations in Alzheimer’s.
Some argue that Alzheimer’s might be more properly understood as a complex failure, caused by the aging of multiple interacting physiological systems.
If so, can we find a root cause? It could very well be. Some of these systems may share an underlying pathology [17]. For example, a strong association between the incidence of Type 2 diabetes (T2D) and Alzheimer’s [18] has led to a desire for a better understanding of the shared pathology of diseases which involve the aggregation of misfolded proteins and a speculation that such diseases may share complex downstream interactions.
Similarly, there is increasing recognition of the role of Endoplasmic Reticulum (ER) stress and dysregulation of ER function in AD pathology. The restoration of ER stress markers seems to prevent amyloid’s toxic effects in mice [19,20].
That the physiology underlying Alzheimer’s involves multiple factors would hardly be surprising in an organ as complex as the brain. It is to be hoped that a greater understanding of systems and disease states in Alzheimer’s will lead to new targets to treat.
Pros
Yet an increased understanding of the potentially multifactorial interactions in Alzheimer’s has also lent some further support to secretase as the best target for treating Alzheimer’s. This may even yet suggest more gentle modulatory approaches to their inhibition.
In support of the importance of the amyloid hypothesis, the most significant high-profile discovery of recent years has been the identification of a protective mutation in APP in an Icelandic population which significantly reduces BACE1 cleavage of APP [21]. This discovery was seen as providing proof of principle that reducing the amyloidogenic processing of APP has a protective effect.
Further support for the amyloid hypothesis was demonstrated with the recent development of a novel three-dimensional human neural culture model of Alzheimer’s. It was nicknamed, "Alzheimer’s-In-A-Dish". It inhibited Aβ generation using β- or γ-secretase inhibitors. It not only reduced Aβ deposition but also attenuated the generation of aggregated phospho-tau [22]. Although this is only a single cell system, it further highlights the potential of β- and γ-secretase inhibitors. This adds weight to the hope that highly selective therapeutics with minimal side effects still may have potential to treat AD.
One major issue that has been highlighted by the failures of so many AD clinical trials is the design of the trials themselves. It is now generally accepted that a large number of the clinical trials of AD treatments may have failed due to the patients’ being too far advanced in the disease process to see any clinical effect from a potential therapeutic. Amyloid deposition in AD is now thought to begin many years before the appearance of cognitive symptoms and ultimate diagnosis of dementia [23]. Much drug development in AD is now beginning to focus on the targeting of patients at the very early stages of the disease, before obvious dementia, particularly in groups with familial AD. As such, the FDA have produced guidance for the design of clinical trials involving patients who do not present with overt dementia. It will be interesting to see whether some of the previously failed drugs will have clinical efficacy when executed in newly designed clinical trials.
One consideration is that if healthy people are to be subjects in the drug discovery process, the prospect of ongoing problems with side effects could become even more serious. It is not yet clear what level of secretase reduction may be required to achieve sufficient reduction in brain Aβ and whether this will bring with it an acceptable level of side effects. For example, the suspension of the drug trial for LY2886721 due to liver toxicity was initially assumed to be due to off-target effects that might be fixed by making adjustments to the drug. However, worryingly, it was recently suggested that the side effect may have come about due to the chemical in the body that the drug fixed, having important functions that may extend beyond the brain [24]. Therefore, it might have been the very same chemical curing the brain while killing the liver.
The Future
Proponents of the amyloid hypothesis argue that the clinical trial data to date has not yet adequately tested the hypothesis; it is not yet clear whether trial failure was due to failure of the amyloid hypothesis itself or rather, to:
- Ineffective late intervention
- Drug side effects masking effects on cognition
- Inadequate engagement of the target secretases by the drugs.
The outcomes of current "Earlier-Intervention" trials may be key to the continued focus on the amyloid hypothesis as the central focus of Alzheimer’s research.
Also, success in a secretase-targeting early intervention trial which came at a cost of significant side effects in relatively healthy individuals might well lead to a renewed focus on more indirect modulatory approaches to secretase inhibition, and to a further increase in efforts to determine high quality biomarkers for the development of the disease. Despite the recent failures in clinical trials significant hope yet lies around the secretase targeting approach.
On the other hand, more high profile clinical failures could potentially result in the withdrawal of major pharmaceutical companies from the funding of anti-Aβ clinical trials.
Any major success would doubtless be regarded as justification of the effort and resources used in the pursuit of anti-Aβ therapies over the last decade.
Executive summary
| • Alzheimer’s disease (AD) is the most significant disease of the aging population. | |||||
| • As yet there are no available drugs that can halt or even stabilize disease progression. | |||||
| • The amyloid hypothesis describes the accumulation of β amyloid (Aβ) as the event that triggers AD. Aβ is a derivative of the larger amyloid precursor protein (APP). | |||||
| • The roles of α-, β- and γ-secretase play an essential part in this model. | |||||
| • ADAM10 is likely to be the most physiologically relevant α-secretase in neurons. | |||||
| • Because α-secretase processing of APP involves cleavage within the Aβ peptide sequence, precluding Aβ formation, activation α-secretase is considered a potential treatment for AD. | |||||
| • Indirect activation of α-secretase via associated signaling pathways may be the best approach. | |||||
| • BACE1 is the β-secretase enzyme, and competes with α-secretase for APP cleavage. | |||||
| • BACE1 cleaves APP at the N-terminal of the Aβ domain – subsequent cleavage by γ-secretase releases Aβ isoforms. | |||||
| • Inhibition of the β-secretase would block Aβ production and thus represents a therapeutic strategy in AD treatment. | |||||
| • The past decade has seen intense research efforts toward BACE1 inhibition but as yet there have been no successes in clinical trials. | |||||
| • Concerns over potential side effects from this approach have grown with time and increased understanding of the role of this enzyme. | |||||
| • Partial inhibition of BACE1 activity could represent a feasible strategy. | |||||
| • γ-secretase is a protein complex comprising presenilin, nicastrin, APH-1 and PEN-2. It cleaves the APP fragment following β-secretase cleavage to produce Aβ. | |||||
| • As with β-secretase, inhibition of γ-secretase represents an obvious strategy for inhibiting Aβ. | |||||
| • Clinical trials of γ-secretase inhibitors to date have demonstrated unacceptable side effects and lack of positive effect on cognition. This is probably because γ-secretase cleaves a number of important substrates other than APP. | |||||
| • Hope remains around two newer strategies which aim to avoid an important second substrate of γ-secretase called Notch. | |||||
| • It remains far from certain whether targeting the secretases involved in APP processing will yield therapeutic success. | |||||
| • High profile clinical failures have called into question the viability of the amyloid hypothesis. | |||||
| • A number of the clinical trials of AD treatments may have failed due to the patients’ being too far advanced in the in the disease process. Newer trials will target patients at earlier stages of the disease. | |||||
| • The outcomes of such trials may be key to continued focus on the amyloid hypothesis as the central tenet of AD research. | |||||
References
| 1. | Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580), 353–356(2002). |
| 2. | Puig KL, Combs CK. Expression and function of APP and its metabolites outside the central nervous system. Exp. Gerontol. 48(7), 608–611 (2013). |
| 3. | Salloway S, Sperling R, Fox NC et al. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med 370(4), 322–333(2014). |
| 4. | Doody RS, Thomas RG, Farlow M et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 370(4), 311–321 (2014). |
| 5. | Katzman R. Alzheimer’s disease. N. Engl. J. Med. 314(15), 964–973 (1986). |
| 6. | Terry RD, Masliah E, Salmon DP et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30(4), 572–580 (1991). |
| 7. | DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27(5), 457–464(1990). |
| 8. | Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly.Neurobiol. Aging 16(3), 285–298; discussion 298–304 (1995). |
| 9. | McLean CA, Cherny RA, Fraser FW et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46(6), 860–866 (1999). |
| 10. | Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 158(2), 328–337 (1999). |
| 11. | Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563(1–2),311–314 (1991). |
| 12. | Busciglio J, Lorenzo A, Yankner BA. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol. Aging 13(5), 609–612 (1992). |
| 13. | Lambert MP, Barlow AK, Chromy BA et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95(11), 6448–6453 (1998). |
| 14. | Kayed R, Head E, Thompson JL et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300(5618),486–489 (2003). |
| 15. | Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem.272(35), 22364–22372 (1997). |
| 16. | Lesne S, Koh MT, Kotilinek L et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440(7082), 352–357 (2006). |
| 17. | Spinney . Alzheimer’s disease: the forgetting gene. Nature. 5, 510(7503), 26–28 (2014). |
| 18. | Kim I, Lee J, Hong HJ et al. A relationship between Alzheimer’s disease and Type 2 diabetes mellitus through the measurement of serum amyloid-beta autoantibodies. J. Alzheimers Dis. 19(4), 1371–1376 (2010). |
| 19. | Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer’s disease: current approaches and future strategies. Curr. Drug Targets14(1), 114–122 (2013). |
| 20. | Viana RJ, Steer CJ, Rodrigues CM. Amyloid-β peptide-induced secretion of endoplasmic reticulum chaperone glycoprotein GRP94. J. Alzheimers Dis. 27(1),61–73 (2011). |
| 21. | Jonsson T, Atwal JK, Steinberg S et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488(7409), 96–99(2012). |
| 22. | Choi SH, Kim YH, Hebisch M et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515(7526), 274–278 (2014). |
| 23. | Sperling RA, Aisen PS, Beckett LA et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7(3), 280–292 (2011). |
| 24. | The Human Protein Atlas, Base I: www.proteinatlas.org/ENSG00000186318-BACE1/tissue. |